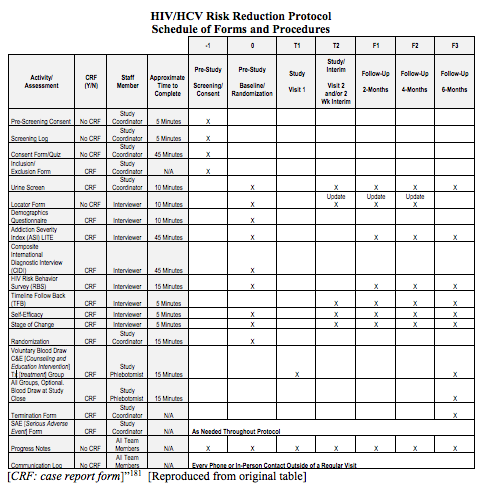
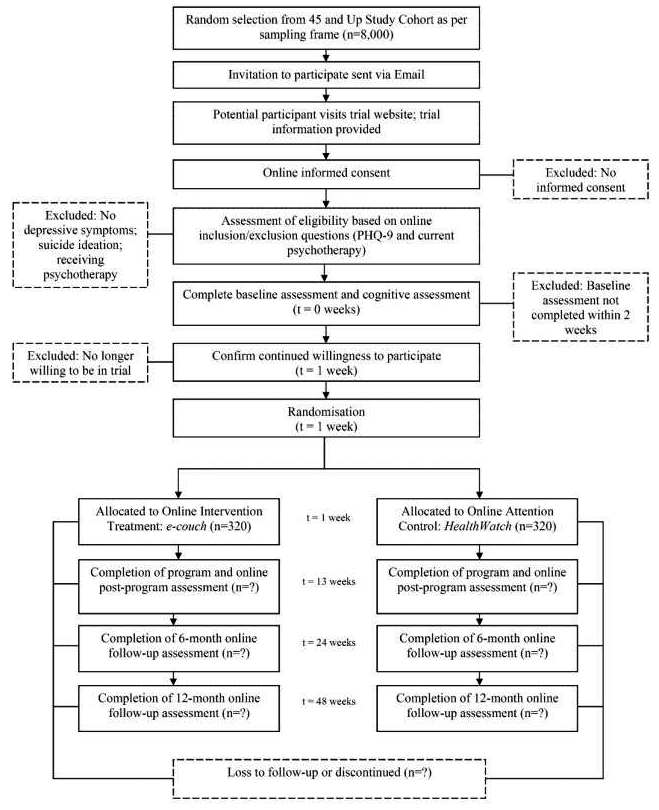
Item 13: Time schedule of enrolment, interventions (including any run-ins and washouts), assessments, and visits for participants. A schematic diagram is highly recommended (see Figure 1).
Example 1
“The main outcomes of interest are the drug and sex-related HIV [human immunodeficiency virus] and HCV [hepatitis C virus] risk behaviors . . . Clients will be assessed using the full battery of instruments from the Common Assessment Battery (CAB), along with the Self-Efficacy and Stages of Change questionnaires and a Urine Drug Screen after consenting . . . questionnaires will take place for all participants 14-30 days after randomization during which they will be given the Stages of Change and Self-Efficacy questionnaires, the Timeline Follow-Back, and a UA [urinalysis]. Follow-up interviews, using the full battery (CAB and questionnaires), will be collected at 2 months (56 days), 4 months (112 days) and 6 months (168 days) after the randomization date. A 14 day window, defined as 7 days before and 7 days after the due date, will be available to complete the 2 and 4 month follow-up interviews and a 28 day window, defined as 7 days before and 21 days after the due date, will be available to complete the 6 month follow up interview . . .
7.1.1 Common Assessment Battery (CAB)
A Demographic Questionnaire . . .
The Composite International Diagnostic Interview Version 2.1 . . .
The Addiction Severity Index-Lite (ASI-Lite) . . .
The Risk Behavior Survey (RBS) . . .
7.1.2 Additional Interviews/Questionnaires
To assess drug use, urinalysis for morphine, cocaine, amphetamine, and methamphetamine will be performed at the 2-Week Interim Visit, and the 2-, 4-, and 6-month Follow-up visits . . .
Stage of change for quitting drug use will be measured using a modification of the Motivation Scales . . .
Example 2
” 182 [Click to enlarge]
Explanation
A clear and concise timeline of the study visits, enrolment process, interventions, and assessments performed on participants can help to guide trial conduct and enable external review of participant burden and feasibility. These factors can also affect the decision of potential investigators and participants to join the trial (Item 15).91
A schematic diagram is highly recommended to efficiently present the overall schedule and time commitment for trial participants in each study group. Though various presentation formats exist, key information to convey includes the timing of each visit, starting from initial eligibility screening through to study close-out; time periods during which trial interventions will be administered; and the procedures and assessments performed at each visit (with reference to specific data collection forms, if relevant) (Figure 1).
| 12: Outcomes | 14: Sample size |