Item 20a: Statistical methods for analysing primary and secondary outcomes. Reference to where other details of the statistical analysis plan can be found, if not in the protocol.
Example
“The intervention arm (SMS [short message system (text message)]) will be compared against the control (SOC [standard of care]) for all primary analysis. We will use chi-squared test for binary outcomes, and T-test for continuous outcomes. For subgroup analyses, we will use regression methods with appropriate interaction terms (respective subgroup × treatment group). Multivariable analyses will be based on logistic regression . . . for binary outcomes and linear regression for continuous outcomes. We will examine the residual to assess model assumptions and goodness-of-fit. For timed endpoints such as mortality we will use the Kaplan-Meier survival analysis followed by multivariable Cox proportional hazards model for adjusting for baseline variables. We will calculate Relative Risk (RR) and RR Reductions (RRR) with corresponding 95% confidence intervals to compare dichotomous variables, and difference in means will be used for additional analysis of continuous variables. P-values will be reported to four decimal places with p-values less than 0.001 reported as p < 0.001. Up-to-date versions of SAS (Cary, NC) and SPSS (Chicago, IL) will be used to conduct analyses. For all tests, we will use 2-sided p-values with alpha = < 0.05 level of significance. We will use the Bonferroni method to appropriately adjust the overall level of significance for multiple primary outcomes, and secondary outcomes.
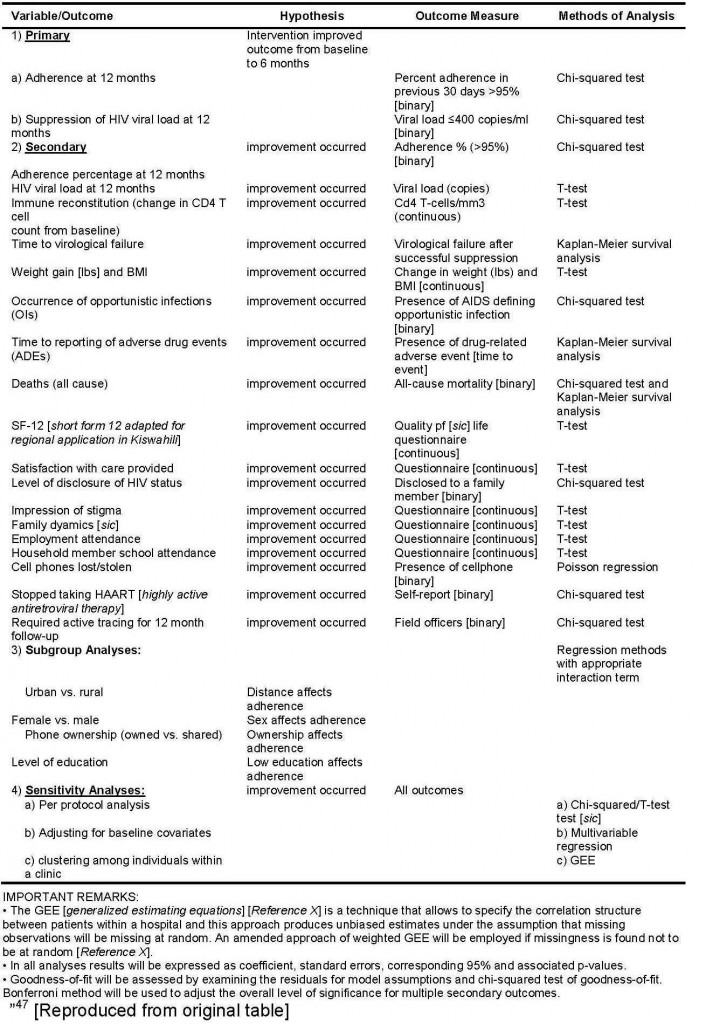
To assess the impact of potential clustering for patients cared by the same clinic, we will use generalized estimating equations [GEE] assuming an exchangeable correlation structure. Table 3 provides a summary of methods of analysis for each variable. Professional academic statisticians (LT, RN) blinded to study groups will conduct all analyses.
Table 3: Variables, Measures and Methods of Analysis
Explanation
The protocol should indicate explicitly each intended analysis comparing study groups. An unambiguous, complete, and transparent description of statistical methods facilitates execution, replication, critical appraisal, and the ability to track any changes from the original pre-specified methods.
Results for the primary outcome can be substantially affected by the choice of analysis methods. When investigators apply more than one analysis strategy for a specific primary outcome, there is potential for inappropriate selective reporting of the most interesting result.6 The protocol should pre-specify the main (“primary”) analysis of the primary outcome (Item 12), including the analysis methods to be used for statistical comparisons (Items 20a and 20b); precisely which trial participants will be included (Item 20c); and how missing data will be handled (Item 20c). Additionally, it is helpful to indicate the effect measure (e.g., relative risk) and significance level that will be used, as well as the intended use of confidence intervals when presenting results.
The same considerations will often apply equally to pre-specified secondary and exploratory outcomes. In some instances, descriptive approaches to evaluating rare outcomes – such as adverse events – might be preferred over formal analysis given the lack of power.300 Adequately powered analyses may require pre-planned meta-analyses with results from other studies.
Most trials are affected to some extent by multiplicity issues.301;302 When multiple statistical comparisons are performed (e.g., multiple study groups, outcomes, interim analyses), the risk of false positive (type 1) error is inflated and there is increased potential for selective reporting of favourable comparisons in the final trial report. For trials with more than two study groups, it is important to specify in the protocol which comparisons (of two or more study groups) will be performed and, if relevant, which will be the main comparison of interest. The same principle of specifying the main comparison also applies when there is more than one outcome, including when the same variable is measured at several time points (Item 12). Any statistical approaches to account for multiple comparisons and time points should also be described.
Finally, different trial designs dictate the most appropriate analysis plan and any additional relevant information that should be included in the protocol. For example, cluster, factorial, crossover, and within-person randomised trials require specific statistical considerations, such as how clustering will be handled in a cluster randomised trial.
| 19: Data management | 20b: Additional analyses |